Even when the movements associated with tardive dyskinesia (TD) are subtle, they can have a significant impact on patients’ lives and interfere with the management of underlying mental health conditions. Uncovering the presence and consequences of TD, no matter the severity, can lead to appropriate treatment of patients with this disorder.
TD is a persistent, typically irreversible, hyperkinetic movement disorder resulting from chronic exposure to antipsychotics and other dopamine receptor blocking agents (eg, antiemetics or prokinetics).1-3 In the United States, TD affects approximately 785,000 patients.4 In truth, though, TD may be underdiagnosed and undertreated. Only around 15% of patients with TD receive a formal diagnosis, and fewer than 6% of patients are treated with vesicular monoamine transporter 2 (VMAT2) inhibitors, the only medication approved by the US Food and Drug Administration for TD.4 In a study of patients with probable TD, less than half were formally diagnosed, despite having lived with the impact of TD for 5 years.4
The impact of TD, even when movements are mild, reaches into many aspects of patients’ lives. This is reflected in the IMPACT-TD Registry study, an ongoing, real-world, longitudinal study evaluating the impact of TD on patients.5 In an interim analysis of clinician-reported data from 286 patients, 98% of patients experienced the impact of TD on some aspect of their lives.5 Importantly, more than half of patients with mild TD experienced moderate to severe impacts on social, psychological, physical, and vocational areas of life. In fact, 54% of patients with very mild TD, as assessed by the Clinician’s Global Impression of Severity, and 61% of patients with mild TD experienced moderate to severe impact.5
A challenge in evaluating the impact on patients is that clinicians might perceive mild TD movements differently from how patients experience them.5 This contrast is exemplified below, where a patient describes the impact of mild TD with and without sound. Without audio, simply watching the patient’s movements might not indicate any impact, but when sound is included, the significant impact becomes clear (see Videos 1 and 2).
(no sound)
The visual assessment of TD is mild
(with sound)
The verbal description of TD indicates significant impact
Patient images used with permission.
The IMPACT-TD Registry study also showed that approximately 74% of patients with an Abnormal Involuntary Movement Scale score of 1 to 6 experienced a moderate to severe impact of TD on their lives.5 The impact of TD was particularly acute in the psychosocial aspects of life (eg, embarrassment in social situations, impacting the ability to enjoy things they do for fun) (see Video 3).6
Patient images used with permission.
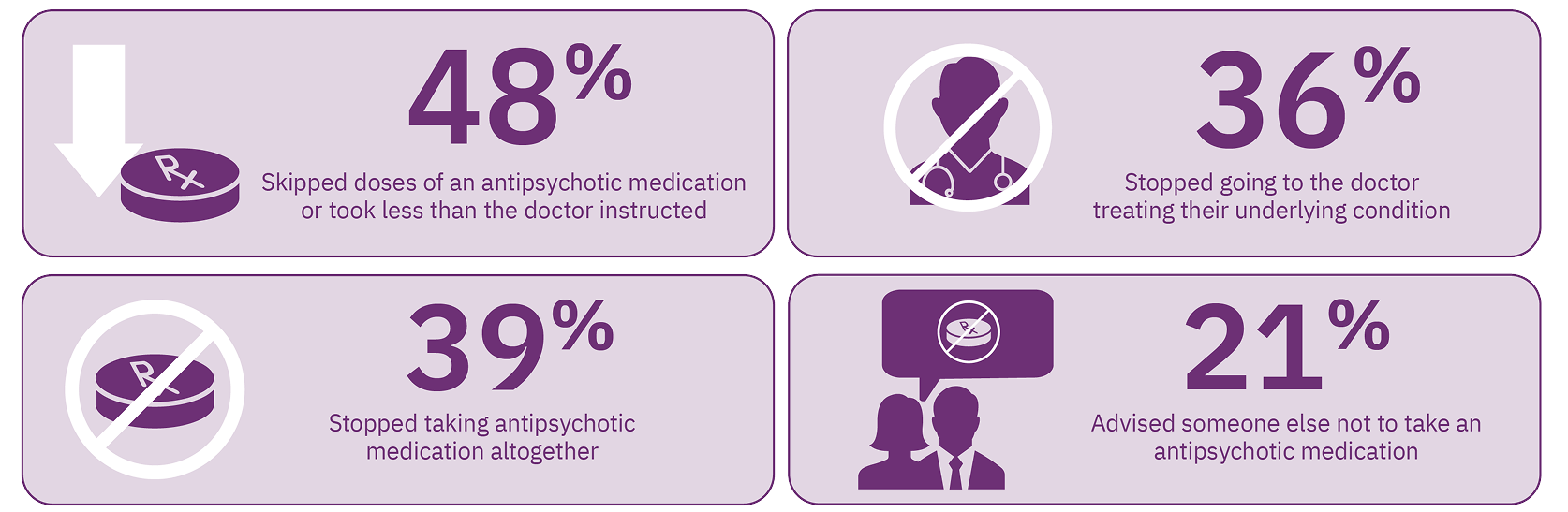
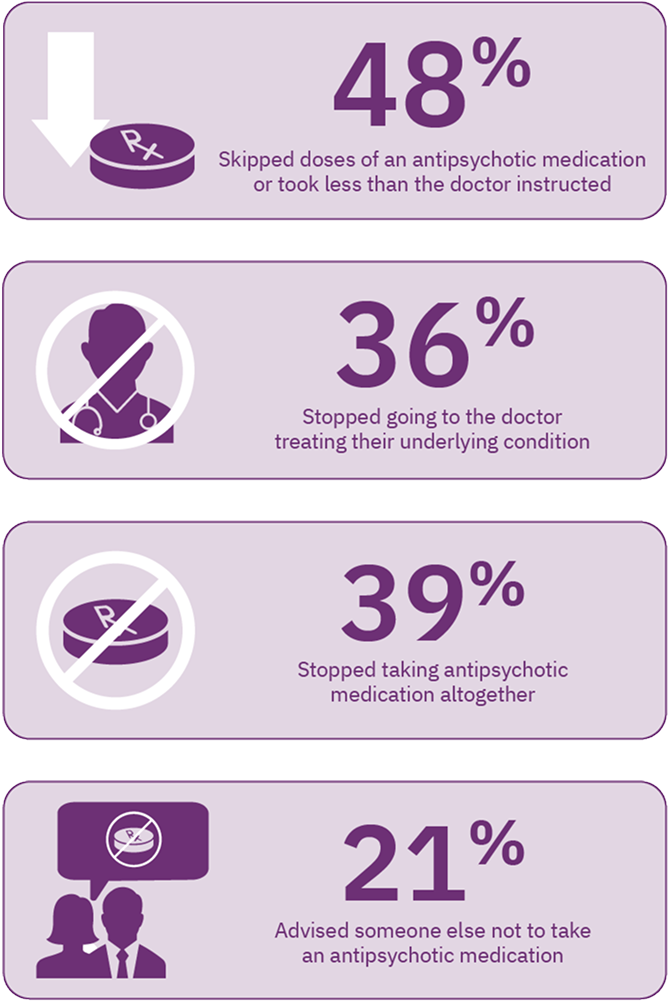
TD may also adversely affect the clinician’s ability to manage the patient’s underlying mental health condition. In an online survey of 269 patients, nearly 50% of patients reported that because of TD, they skipped doses of their antipsychotic medication, and nearly 40% stopped taking their antipsychotic altogether. Over 20% advised others not to take an antipsychotic medication. Moreover, 36% of patients reported they stopped going to the doctor to treat their underlying condition due to TD (Figure 1).7 The experiences reported by these patients demonstrate the difficulty of managing underlying mental health conditions when TD is present.
The American Psychiatric Association (APA) recommends treating patients when their TD negatively impacts them, regardless of severity.8
Unfortunately, clinicians don’t always have a full picture of the impact of TD on their patients. In one online survey, 154 patients with TD and 150 clinicians were asked to rate the impact of TD on 4 domains; psychological/emotional, physical, professional, and social. Patients reported a greater awareness of the impact of TD than clinicians. While patients and clinicians both reported similar impact of TD in the psychological and emotional domain, patients reported a significantly greater level of impact across all domains compared with clinicians. Nearly twice as many patients reported their physical domain was impacted as clinicians. These data highlight the need for increased dialogue between patients with TD and their clinicians to assess the impact of TD on patients.9
What can clinicians do to have a deeper understanding of the impact of TD on the lives of their patients? Once a patient has been diagnosed, it is important to ask about the impact at every clinical visit.10 Consider identifying the consequences of TD across the 4 domains: psychological/psychiatric, social, physical, and vocational/educational/recreational.11 Talking with caregivers, family, and friends may also be helpful in identifying the impact of TD.11 Specific questions related to each domain may include the following11,12:
- How bothered are you by negative reactions to your TD by your friends or family?
- Have you experienced feelings of low self-esteem or embarrassment because of your TD?
- Do you have trouble eating or falling asleep?
- How often does your TD impact your ability to perform your job?
Consider the Impact-TD scale as a guide in clinical practice to assess the impact of TD. Click here to access.
There are many reasons why it is important for you, as a healthcare professional, to routinely assess the impact of TD in your clinical practice. First, TD can affect your ability to manage your patients’ ongoing mental health conditions.7 In addition, TD can profoundly impact your patients, influencing their ability to perform daily functions, be productive, and socialize.5 Finally, the APA Guidelines recommend treating patients with TD when the condition negatively impacts them, even if the movements are mild.8 Routine assessment of both the presence and consequences of TD, through careful questioning and listening, can illuminate the impact of TD on your patients and lead to appropriate treatment.11,12